UK MHRA expects “high volume” side effects (ADR) but “vaccine” is “safe and efficient”
The UK urgently provisioned outside EU procurement standards for £1.5M an AI software to process the expected high volume of Covid-19 vaccine Adverse Drug Reaction (ADRs).

As we are being told there were no corners cut in assessing the safety of Pfizer experimental vials (“vaccine”) and people’s health is paramount, the European Federation of Pharmaceutical Industries and Associations deploys a far more cautious approach.
We would like to share the following two articulations.
A/ First part
Perhaps you read in FT.com “The speed and scale of development and rollout do mean that it is impossible to generate the same amount of underlying evidence that normally would be available through extensive clinical trials and healthcare providers building experience,” reads a memo circulated to members by Vaccines Europe, a division of the European Federation of Pharmaceutical Industries and Associations.
The leaked document FT obtained says that this creates “inevitable” risks.
B/ Second part
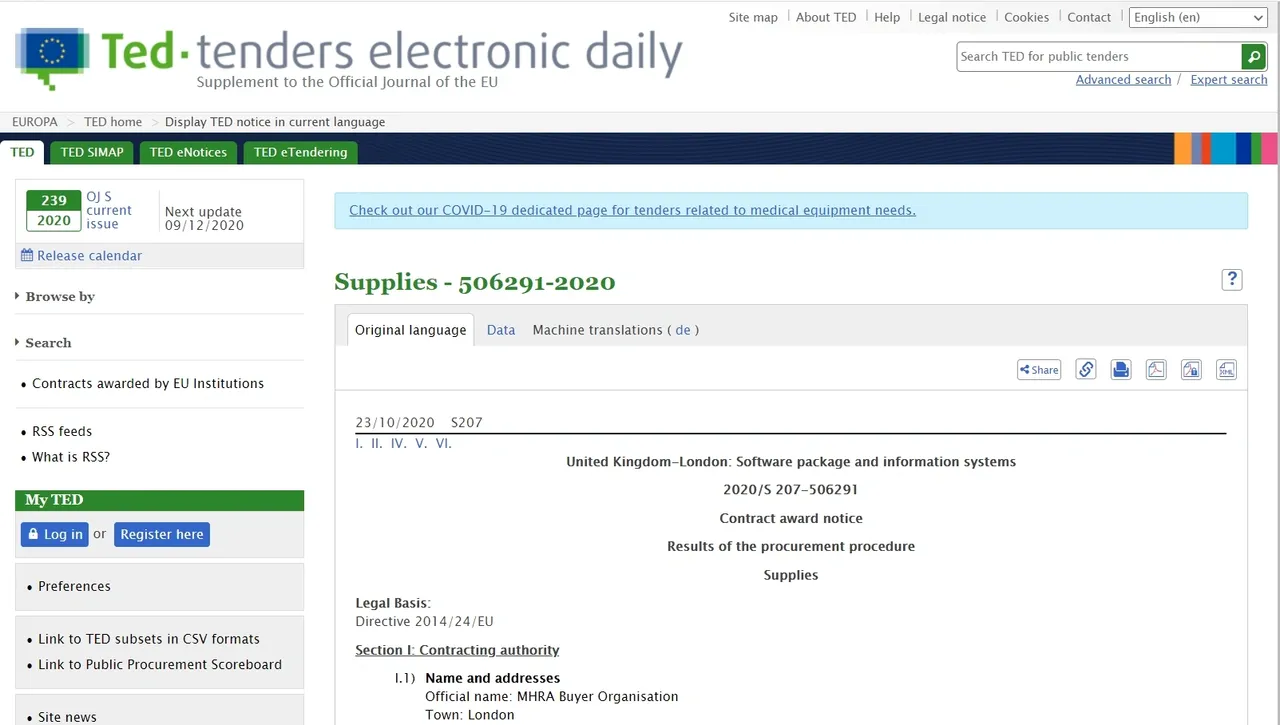
EU Tender short description (ted.europa.eu/ ). “Supplies – 506291-2020”, value £1,5M, Procurement falling outside the scope of application of the directive for “reasons of extreme urgency (,…) related to the release of a COVID-19 vaccine”: “The MHRA urgently seeks an Artificial Intelligence (AI) software tool to process the expected high volume of Covid-19 vaccine Adverse Drug Reaction (ADRs) and ensure that no details from the ADRs’ reaction text are missed.”
Source: https://lnkd.in/eJsy2Ms
It means, and please tell us how it could be otherwise if you think so, the UK government on one side pushes the certification and the large-scale diffusion of Covid vaccine saying it fits all health and safety standards, while on the other hand, knows it is expected a ‘high volume of Covid-19 vaccine Adverse Drug Reaction (ADRs)”.
As the only currently released vaccine is the Pfizer one, the Adverse Side Effects tracking is all about it.
Pfizer test data is not available publicly unless we missed something (let us know).
Thank you for your time and interest.
Please join us by subscribing to our Blog. Posts are occasional and written as thoughts come.
Please leave your comment at the bottom of this page to continue the reflection on this post.
If you are looking for a reliable, independent professional consultancy to assist you in getting through the mist and the storm and cutting through an often artificial complexity, please do get in touch with us for an informal discussion, or write to contact @ reasonmakesense .com (please remove spaces)
Get in touch to discuss freely; reason will Make Sense, with you and for you.
A few words about Reason

reason supports Shareholders, Board, C-Suite Executives and Senior Management Team in achieving Business Excellence and Sustainability through our Praxis unique approach.
We Make Sense with you and for you.
We work and think with integrity, are independent and fed by a very broad spectrum of robust information sources, which is certainly one of the rarest and best qualities a consultancy can offer demanding decision-makers willing to overcome challenges and reach impactful, tangible and measurable Business Excellence.
We follow reason, facts, best practices, common sense and proper scientific approaches. This is our definition of professionalism. It brings reliability, confidence and peace of mind.
Please check our offering, subscribe directly on this page, write to contact @ reasonmakesense .com (without the spaces) or click on our logo below to get redirected to our contact form.
Thank you for reading!
Reason Praxis | Make Sense
Excellence & Sustainability
www.reasonmakesense.com
There is nothing wrong in doing things right, first time.
Share this post